What Is a Formulary?
In its simplest form, a formulary is a list
A drug formulary is a preferred drug list. The list of medications on the formulary changes based on new evidence-based medicine, the medical decision-making of doctors, pharmacists, and clinicians.
A formulary is dynamic
According to the AMCP (Academy of Managed Care Pharmacy) a formulary is not static, it is dynamic. Because of the dynamic nature of drug development and research, formulary lists are dynamic. A formulary is described by AMCP as:
“…An ongoing process whereby a health care organization, through its physicians, pharmacists, and other health care professionals, establishes policies on the use of drug products and therapies and identifies drug products and therapies that are the most medically appropriate and cost-effective best to serve the health interests of a given patient.” [i]
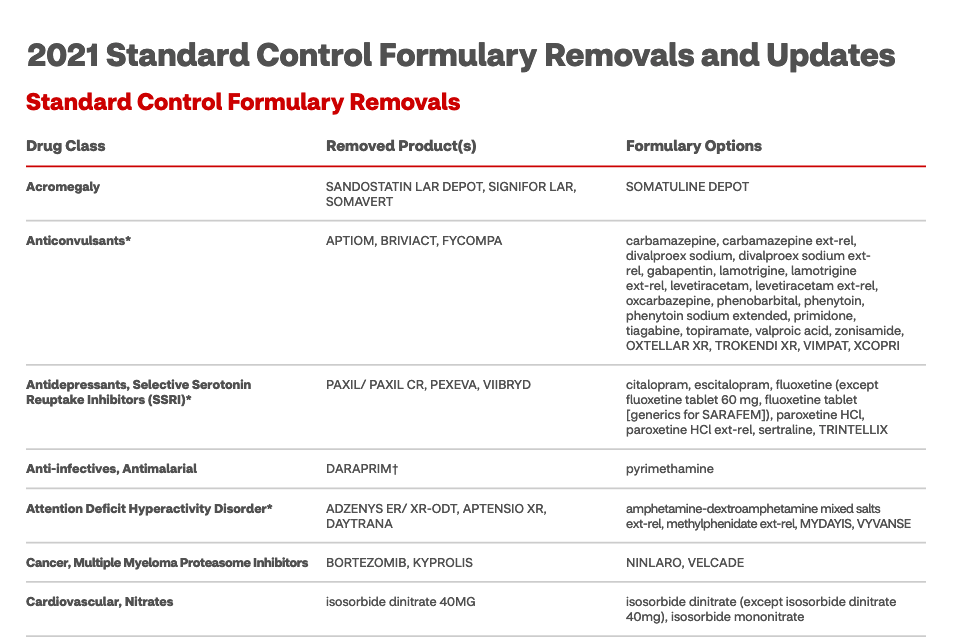
Understanding formulary drugs and non-formulary drugs, (also known as off formulary drugs) is as simple as viewing a current formulary list, or as complex as some of the topics below. I will summarize a few points for you below.
Formulary lists are driven by policy, not by medical codes
Formularies are not driven by billing codes. Instead, the drugs chosen to be offered by a health plan (also called a ‘payer’) are those medications that a panel of independent medical experts on a pharmacy and therapeutics team (P&T) have selected based on evidence of the drug’s efficacy and price. HCPCS codes[ii] apply when a physician administers the drugs but do not apply when the patient self-administers the drug and does not claim insurance to cover the cost.[iii]
Reading formulary listings
Sometimes, formulary symbols are used to refer to the following meanings:
- C = Covered,
- EXCL = Excluded Drug
- NF = Non-formulary
- NP = Not preferred
- NR = Not reimbursable
- ONC = Oncology Program
- P = Preferred PDL
- PA = Prior Authorization
- PDL = DHS’ Preferred Drug List
- PX = Preferred at level X, with X indicating a more preferred medication or supply. This will show as “P24” for a level 24 medication or supply. Higher numbers indicate higher preference.
- QL = Quantity Limit, Age = Age Edit
- SP = Specialty Drug,
- ST = Step Therapy
- U = Unknown formulary status
NOTE: These are examples. Formulary listings, symbols and codes usually vary by PBM and payor
Formularies should serve multiple purposes
Formularies should if properly structured, serve multiple purposes, including:
- encouraging the use of safe and effective medications based on clinical guidelines
- controlling cost and insurance utilization
What is a PBM?
Pharmacy benefit managers (PBMs) typically negotiate discounts and rebates with pharmaceutical manufacturers on behalf of their clients—insurers, employer groups, and other payers—to create and manage prescription medicine formularies.[iv]
Who are the Largest PBMs?
The PBM market is highly consolidated, with just 3 PBMs—CVS Caremark, Express Scripts, and OptumRx—managing the pharmacy benefits for approximately 256 million Americans.[v] In 2019, these 3 PBMs handled 74% of all prescriptions processed in the U.S.[vi]
What Influence do PBMs Have over the Access to and Cost of Drugs?
PBMs have significant influence over drug cost, supply and access and because the formularies are set by PBMs, even the drugs that apear as options in e prescribing systems in Electronic Health Records (EHRs). This tends to influence physician behavior regarding what medications they prescribe. PBMs do not actually supply drugs, but they are central to the flow of funds between drug manufacturers, pharmacies, health plans, drug wholesalers, hospitals, and physicians. PBM negotiated discounts and rebates, as well as formulary placements, are negotiated by PBMs. For more regarding the flow of funds and flow of drugs, see this article.
Health Plans Often Outsource Formulary Management to Pharmacy Benefit Managers (PBMs)
Because of the complexity and numerosity of drugs, new FDA approvals, and ongoing industry research, health plans may outsource formulary management and coverage determinations and pharmacy benefit management companies (PBMs) as a third-party, or they may own or acquire a PBM to perform formulary management functions for the plan.
Because Formularies are Dynamic, Patients may Experience Changes in Access to Drugs that are Managed by PBMs and their Formularies
Patients with insurance may have limited or curtailed access to certain drugs based on several methods used by PBMs and formulary policy committees. These include:
- Formulary tiers (some formularies may have three tiers, four tiers, five tiers or six tiers)
- Step therapy
- Open vs. closed formularies
- Pharmacy & Therapeutics (P&T) Committee Review Policy Updates
- Generic Substitution
- Therapeutic Alternates
- Therapeutic Interchange
- Therapeutic Substitution
- Drug Utilization Review (Drug Use Review, DUR, and Drug Use Evaluation)
- Formulary Exclusions
There is an appeals process, discussed in another article that provides insured patients with a method to appeal a decision not to cover a drug that is not on a formulary.
PBM Policy May Vary Regarding Access to Drugs not on a Formulary
Health plans may provide physicians, and their patients access to non-formulary drugs where medically necessary.
Ethical Standards for Patients / Healthcare Consumers Regarding PBMs and Formularies
Notably, the Principles of a Sound Drug Formulary System provide that PBMs and the formularies they manage should: [vii]
- “Provide a rationale for specific formulary decisions when requested. The formulary system should include a well-defined process for the physician or other prescriber to use a non-formulary drug when medically indicated.”
- “Enable individual patient needs to be met with non-formulary drug products when demonstrated to be clinically justified by the physician or other prescriber.”
- “Institute an efficient process for the timely procurement of non-formulary drug products and impose minimal administrative burdens.”
- “Provide access to a formal appeal process if a request for a non-formulary drug is denied.” (See Formulary Exceptions)
- “Include policies that state that practitioners should not be penalized for prescribing non-formulary drug products that are medically necessary.” [viii]
One of the challenges of the ethical guidelines is that in our opinion they presume a thoughtful and sometimes protracted appeals process. If a drug is needed on an emergent basis and not covered by insurance, or if a prior authorization is required, a physician may need to forgo prior authorization and opt to treat (or, be required to treat) a patient to stabilize them under EMTALA
Related Articles
Drug Distribution, Drug Pricing, and Drug Classification Systems
Pharmacy Benefit Managers, Drug Pricing and Appeals
How do Pharmacy Benefit Managers Determine Pricing?
Pharmacy Benefit Manager Expert Witness
Knox-Keene Act, Drug Formularies and Pharmacy Benefit Managers in California
Citations
[i] AMCP (Academy of Managed Care Pharmacy)
[ii] Centers for Medicare and Medicaid (CMS) The Healthcare Common Procedure Coding System (HCPCS) is divided into two principal subsystems, referred to as level I and level II of the HCPCS.
[iii] What are HCPCS J Codes.
[iv] Xcenda, AmerisourceBergen. Skyrocketing Growth in PBM Formulary Exclusions Raises Concerns About Patient Access
[v] Webster K.:
CVS Caremark limits the purchase of essential medication to avoid stockpiling. Employer Benefit News.
Express Scripts. Daring to imagine a better health care system.
OptumRx. Pharmacy benefit management solutions.
[vi] Fein A. The 2020 economic report on U.S. pharmacies and pharmacy benefit managers. Drug Channels Institute. March 2020.
[vii] American Society of Health-System Pharmacists (ASHP). Principles of a Sound Drug Formulary System. “The endorsement of this document was reviewed in 2011 by the Council on Pharmacy Practice and by the Board of Directors and was found to still be appropriate.”
[viii] Id.