Pharmacy Benefit Manager Drug Pricing and MAC Appeals – PBM MAC Appeals
Pharmacy Benefit Managers drug pricing uses several metrics and Standards. Pharmacy Benefit Managers (PBMs) may use Maximum Allowable Cost or “MAC” pricing on multi-source generics to avoid being overcharged. Establishment of a MAC allows payers to pay the same price for a drug, no matter who manufacturers it. Newer generic products, compared to older generics, may not have as favorable of a spread, thus the need for MAC. It is important to understand the contractual provisions regarding how MAC is determined with specific pharmacies and PBMs and to them perform analytics with full data elements available to determine whether the PBM has performed in adherence to the contractual provisions. To perform PBM MAC appeals it is important to understand how PBMs work, the MAC definition, how to source data to analyze adherence to MAC, and what data must be produced for most PBM MAC appeals.
PBM Fundamental Concepts
Pharmacy Benefit Managers (PBMs) evolved over a few decades in the drug pricing business and intersect the flow of physical drugs, flow of funds, and medical necessity determinations for health plans.
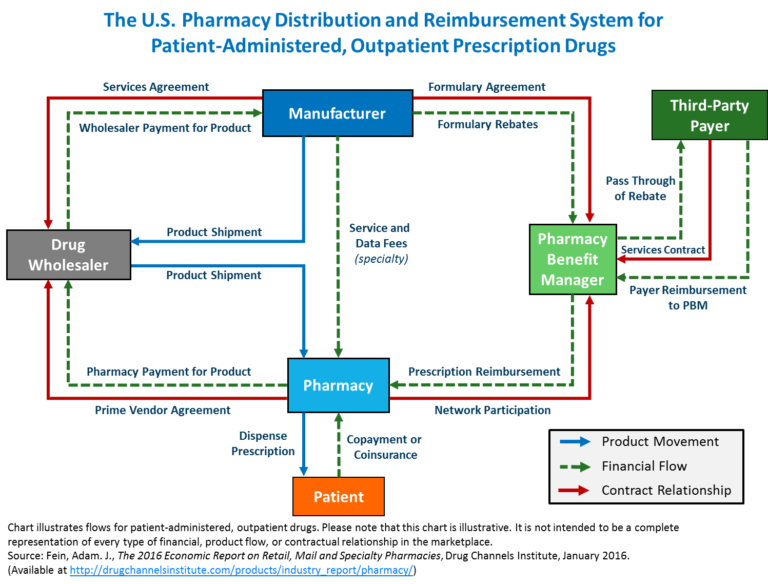
Pharmacy Benefit Manager Flow of Funds
PBMs collect payments from health plans and receive share of rebates from manufacturers are issued back to the health plan. PMBs manage a complex task in the drug supply and beneficiary reimbursement segment of health care. Pharmacy Benefit Managers drug pricing has several facets.
Pharmacy Benefit Manager experts must understand the flow of funds. The Drug manufacturers issue negotiated discounts and rebates for drugs based on volume, market share, formulary placement). PBMs issue contracts to health plans, generally pricing their coverage for drugs at WAC. WAC can be used correctly or it can be manipulated, based for example on last in first out (LIFO) or first in first out (FIFO) pricing. The purchase / payment lots can vary from a small volume of drugs at high price point, or the same drug purchased in high volume at a lower price point. If Pharmacy Benefit Managers contract for a LIFO based WAC they can purchase in high volume at low price to decrease the price point, then purchase a small amount at high price and charge based on LIFO. This can yield artificially high profits. This is not to say that all PBMs operate in this manner, but the use of analytics combined with a detail knowledge of drug classification taxonomies is essential to determine if contractural arrangements are being met.
Appealing MAC Pricing Upon Reimbursement from a Pharmacy Benefit Manager
MAC pricing continues to be one of a highly challenging issue for independent pharmacies because it is a) one of the primary ways in which PBMs reimburse independent pharmacies in the United States, and b) MAC price lists are created entirely by PBMs and kept confidential by PBMs.
In some jurisdictions such as California(see AB 627 (*)) and Georgia, if there is a successful MAC appeal:
- The PBM must adjust the MAC price appealed effective on the day after the appeal is decided;
- PBMs should apply the adjusted MAC pricing to all similarly situated pharmacies;
- A PBM should permit a pharmacy that successfully appeals to rebill / reconcile with the PBM for any financial difference in the claim appealed.
If an appeal is denied, the PBM must provide the reason for the denial.
Data Quality for Analytics to Support PBM MAC Appeals
It is essential for a any entity that has a PBM contract to have good data records, maintained in Standard formats. Generally a MAC appeal requires completing a form that includes these elements. Forms and data elements may vary by payor. Pharmacy Benefit Managers drug pricing using MAC for an appeal may require solid data including but not limited to:
- Appeal date
- Contact information
- NCPDP number
- NDC number
- GPI number
- Pharmacy name
- Rx#
- Fill date
- Quantity
- Acquisition cost
- Invoice number
Another payer lists these items as pertinent for a MAC appeal:
- Filled Date
- BIN
- PCN
- NCPDP
- RX #
- NDC
- Compound
- Reason for Appeal
Data type and data quality are essential in filing appeals. One payer lists these points regarding the data formats, states that these are mandatory, and that it will reject any appeal that does not contain them as follows:
Reason for Review
- MAC Unit is below cost
- Drug is experiencing supply issues, please review MAC.
- Dispensed least expensive generic
- Other – Please use the notes section to explain
Compound Y/N
- Y (select Y to indicate a compound)
- N (select N to indicate a non-compound)
- If more than one ingredient is to be reviewed fill out individual lines for each
- NDC.
PBM MAC Appeal State Standards and Legislation
* According to the California Pharmacists Association, AB 627 for PBM MAC Appeals was developed through negotiations with stakeholders. The California Pharmacists Association worked with multiple PBMs and health plans to arrive at mutually agreed upon Standards to address pharmacists’ concerns in a way that is acceptable to the needs of the PBMs and health plans. The provisions in this bill closely mirror those of bills in other states that PBMs have also agreed to.
Related Topics
Drug Pricing Expert and Drug Classification Systems
I. In healthcare the intersection of demand, price, quality and sources of suppliers alone does not determine whether pricing is appropriate. Medical necessity, a complex method of determining whether a product or service should be used is also a factor. Medical necessity is used by payors, including private insurance, Medicare and Medicaid to determine whether a medical procedure should be provided at all. (See Drug Pricing Legislation and Inefficient Markets Theory – No World Borders)
II. Drugs are categorized by over 10 different classification systems, however the National Drug Code (NDC) (see National Drug Code Directory), the Generic Product Identifier (GPI) (see Medi-Span® Generic Product Identifier (GPI)), and RxNorm used extensively for electronic prescribing are important standards. (See http://noworldborders.com/2018/0…), (see also RxNorm Overview)
III. There are three concepts that are important to understand in pharmaceutical / drug pricing. The first is the flow of physical drugs. The second is flow of funds. the third is eligibility determination for coverage of drug costs by insurance.
(for more information see my recent answer that discusses this on Quora at https://www.quora.com/How-do-pharmacy-benefit-managers-determine-drug-pricing/answer/Michael-Arrigo-1)
Formulary Management by PBMs
From 2014 to 2020, the number of medicines excluded by at least one of the three largest PBMs from their standard formularies increased by an average of 34% per year. With this development, those patients who rely on a PBM for access to their medications with commercial insurance may not be able to access the treatment prescribed by their doctor through their normal insurance coverage.
See also: